Blogs
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member
How an Entrepreneur’s Failure Led to a New Path in Auditing
In his blog post, Michael Mills shares the story of a man who became an auditor after an uncommon turn of events.
Quality Of The Future: Proactive Professionals Needed
In a blog post by Ekaterina Potemkina, she emphasizes that quality professionals must be proactive to thrive in the future.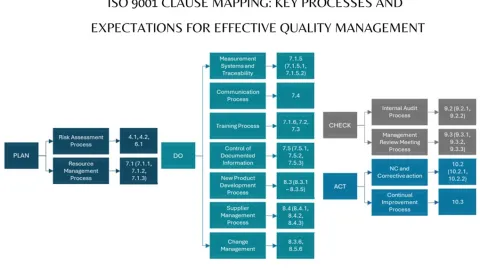
QMS Clause Mapping: Key Processes and Expectations
In his blog post, Suryakant Bhavikatty, Next-Gen eQMS Expert summarize a clause mapping of ISO 9001 by PDCA cyclus in one page.
Conformity Statements and the Challenge of Measurement Uncertainty
According to Jeff Gust, Chief Corporate Metrologist at Fluke Corp, the main issue with conformity statements is that measurements are just estimates of the true value.
Electronic Quality Management Systems Revolutionize Business Operations
According to a personal view by Rahul Saluja of Cyient, electronic quality management systems (eQMS) are transforming how businesses operate.
Have You Used "Five Hows"? The Simple Questioning Technique That Boosts Problem Solving
In his blog post on the Pragmatic Quality Blog, Michael Mills gives his personal take on the 'Five Hows',
Why Quality Management Is Your CIO's Biggest Opportunity
Quality management is becoming a critical opportunity for businesses to improve operations, according to Jerry Foster.
AI is Driving the Shift from Quality Management to Customer Intelligence
According to Paula Kennedy Garcia’s commentary on Forbes,
Avoiding Deadly Diseases of Management to Build a Quality Culture
According to Abdur Rahman Farooq’s opinion, five major challenges are hindering companies from building a strong quality culture.
How to Stay on Top of ISO Certification: Best Practices
According to Erica Smith's blog post on ISO Certification Experts, achieving ISO certification is just the start of an ongoing process.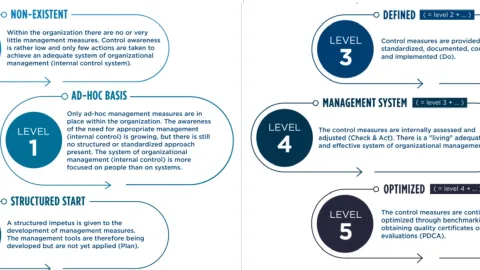
What Your Organizational Maturity Says About You
According to APQC's blog post by Grace Duffy, organizational maturity reflects an organization’s ability to adapt, innovate, and efficiently meet customer needs.
ISO 9001 Revision Pushed to 2026?
ISO 9001’s revision timeline may have hit the snooze button, and according to the blog by Quality Austria, that’s not exactly a surprise.
Counting the Cost: Health and Safety Failures Costing UK Businesses Millions
Health and safety failures are taking a serious toll on UK businesses,
Licensing or Accreditation: The Crucial Choice for New Ventures in Pharma and Medical Devices
When launching Greenfield projects in the pharmaceutical or medical device industries, businesses often face the decision between Good Manufacturing Practice (GMP) licensing and ISO 13485 accreditation.
Process Development and Identification of Critical Process Parameters in Medical and Pharmaceutical Industries
Identifying and controlling Critical Process Parameters (CPPs) is essential to maintaining quality and consistency in medical and pharmaceutical industries, according to Quality Systems Now's blog post.
Benefits Outweigh Challenges in the Implementation of an Electronic Quality Management System
Implementing an electronic quality management system (eQMS) offers a lot of potential for improving processes, according to Quality Systems Now's commentary.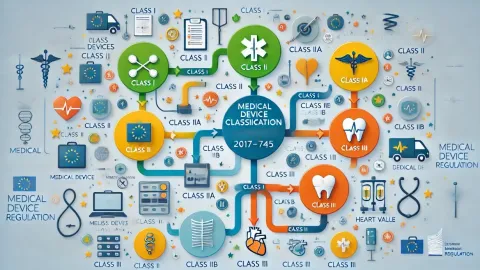
Classification of Medical Devices under EU MDR
Dr. Murugan Kandasamy, Managing Director & CEO at DQS India, Head - Medical Global Support Center, has published a new blog post about Classification of Medical Devices under EU MDR. The most important issues he discusses are:
How to Design a Behavior-Based Training Program
Starting a training program that changes behavior might sound like trying to teach a cat to fetch—possible, but you need the right approach.
Quality Management in construction: ensuring success through rigorous testing
Accoring to Abdur Rahman Farooq, quality management in construction is essential for the safety, durability, and overall success of any project.
"Made in Germany" losing ground as companies look abroad
A commentary by Michael Dunst and Dietmar Vahs points out that the famed "Made in Germany" label is losing some of its sparkle as more German companies consider moving their operations abroad.
Understanding the difference: standards, frameworks, laws, and regulations
Tristan Roth breaks down the often confusing world of compliance by clarifying the roles of standards, frameworks, laws, and regulations.
Problem-solving with the 8D method: a step-by-step guide
In his blog post, MIchael MIlls dissects one of renowned methods to formalize solving a problem.
Story vs. Reality of Quality Managers
Harsh Thakkar from Qualtivate discusses about contrasts common misconceptions about quality managers with their real roles.