Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member
Step-by-Step Guide on How to Audit an Organization for the First Time
Auditing a company for the first time can be both exciting and challenging, according to Soneel Choraria, a seasoned Indian internal auditor currently employed as Internal Audit Manager at Suhail Bahwan, one of the largest business groups in Oman.
The Revision of ISO 9001 Is Confidential, but These Changes Are Likely
Richard King-Davies, Lead Auditor at TÜV SÜD with over 25 years of experience in management systems, explains the ongoing revision of ISO 9001 and what organizations should prepare for.
Stop Viewing Non-Conformities in Certification Audits as Disasters
Non-conformities (NCs) identified during certification audits should not be viewed as failures, argues Mylène Servoles-Doussaint, a consultant specializing in quality, safety, and environmental management systems.
Avoiding Common CAPA Mistakes in Medical Device Quality Management
The CAPA (Corrective and Preventive Action) process for medical devices is at times threatened by common mistakes, but there are strategies to avoid them.
Unlocking Hidden Potential with Overall Equipment Effectiveness (OEE)
Matej Hohnjec, Lean Six Sigma and TRIZ Trainer, explores Overall Equipment Effectiveness (OEE) in this blog post, discussing its role as a critical tool for measuring machine efficiency and addressing common misunderstandings about its use.
Key Requirements for Certification Auditors Under ISO/IEC 17021-1:2015
Tommaso Palmitesta of "Top Quality Consulting" examines the expectations and responsibilities associated with participating in certification audits according to ISO/IEC 17021-1:2015.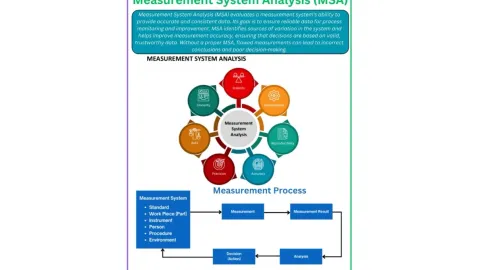
Measurement System Analysis as a Cornerstone of Quality
In a recent blog post, Govind Tiwari, Quality, HSE & Risk Management Expert and Head of Quality at QatarEnergy LNG, highlights the importance of Measurement System Analysis (MSA) in quality management,
Electronic Quality Management Systems Revolutionize Business Operations
According to a personal view by Rahul Saluja of Cyient, electronic quality management systems (eQMS) are transforming how businesses operate.

