Blogs
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member
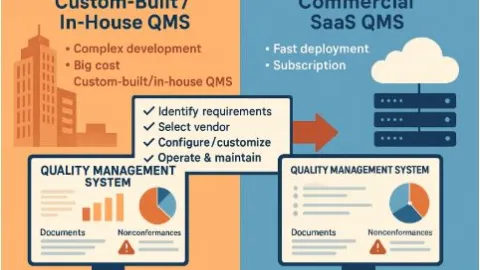
AI Cuts ISO Paperwork in Seconds, Bureaucracy Loses
Artificial intelligence was pitted against bureaucracy to see who would win, showing just how useful AI can be inside a management system.
A Defining Realization: Medical Quality Is the Final Barrier for Patients
Medical quality shifted from being about audits, CAPAs, and deadlines to something far more serious for Dr. Harold E. Braustein when he realized he is the last barrier between a patient and a possible failure.
Auditors Should Care about ISO 42001 Since AI Is Already Inside Audits
Auditors are already reviewing AI-driven decisions without clear rules for who controls them, which is why ISO/IEC 42001 now matters, as pointed out by Jackie Stapleton, Director of Auditor Training Online.
When Details Cloud the View, the Big Picture About Quality Gets Lost
An opinion piece by Michael Mills, a quality expert who uses risk-based thinking to streamline management systems,
PDCA Drives Companies Forward but Certification Bodies Still Lag Behind
Companies put strong effort into Plan Do Check Act (PDCA), but Certification Bodies often do not apply the same approach to themselves, argues Cornelis van Elst, owner of QAssurance and a long-time practitioner working toward real-time food assurance.
ISO Order Falls Short Against Parenting Morning Chaos
Danny Lee, director of OneSystemMgt Ltd and a former Senior Quality Manager in the Royal Air Force,
Avoiding Common Mistakes In IFS Logistics 3 Audits
The most common mistakes in IFS Logistics 3 audits stem from recurring gaps in preparation, and a new post by International Featured Standards (IFS) explains what these issues are and how they can be avoided.
Useful Audits Avoid Nit-Picking and Focus on Context
Auditors should look at what really happens in an organization instead of searching for small paperwork errors,
Revised ISO 10993 Unpacked And Why Industry Is Not Ready For It
The release of the revised ISO 10993-1:2025 , the key standard for the biological evaluation of medical devices, has sparked wide discussion.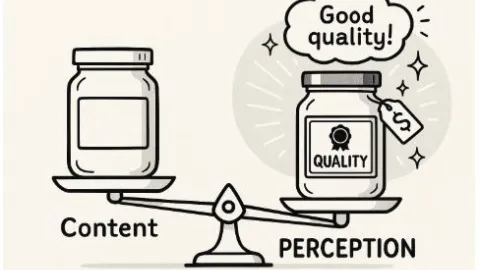
Why Quality Placebo Shapes How A Product Is Judged
A quality product or service is essential, but that alone is not enough, because customers also judge what they buy through signals that shape their expectations and emotions.
How ISO 13485, ISO 14971 and IEC Standards Combine To Build Safer Medical Devices
ISO 13485, ISO 14971 and key IEC safety standards work together as one system to make medical devices safe and compliant.
Why Standardization Fails: “Original Sins” That Turn Certification Into a Lie
Standardization often fails for the same recurring reasons, and a new analysis revisits these “original sins” that weaken quality systems from the start.
Rising Importance of Internet of Things Demands Better Standards and Interoperability
The growing importance of the Internet of Things (IoT) is changing how data is collected, shared and used,
Six Red Flags in Certification Readiness You Should Not Ignore
Certification readiness matters because a management system must work in real operations, not only in documents.
New Revision, Old Mistakes: ISO 9001 Draft Repeats Problems Seen in 2015
The draft of ISO 9001:2026 has triggered strong debate, with many experts warning that it repeats the same unclear wording and weak audit criteria that caused problems in the 2015 revision.
ISO 14060 Moves Net Zero Toward Credible And Practical Action
International standards are emerging as a way to restore trust in net zero claims,
Stricter Global Rules Shaping Pharma and Therapeutic Goods GMP in 2025
As the Quality Systems Now blog post notes, the most important feature of today’s GMP landscape for pharmaceutical and therapeutic goods manufacturers is the impact of stricter global rules, rapid digital change, and complex supply chains,
Expert Offers Guidance to Newcomers: Things I Wish I Had Known at the Start of My Quality Career
Pankaj Bhatia, a quality leader with more than 28 years of experience in medical devices and automotive, shares valuable insider lessons for newcomers to the profession.
Five Steps to Reduce the Burden of Social Audits
A new blog post by Dr. Thijs Willaert, Global Director Sustainability Services at DQS, explains that social audits run more smoothly when companies take simple steps that ease pressure on suppliers and build cooperation.
When the Quality Manager Goes Silent, the System Is Already Failing
Mercy Kagendo, Head of QAFS at Farmer’s Choice Limited and Plant Operations Manager at Choice Meats Limited, shares a personal reflection on what it truly means when a quality manager stops speaking up.
When Reviewers Become Technical Previewers, Audit Impartiality Is Compromised
Cornelis van Elst, Founder of QAssurance, warns in his blog post that impartiality in certification audits is being compromised as technical reviewers become “technical previewers”.
Lost in Translation: Making Quality Speak the Language of Business
Quality professionals often struggle to show their real impact because they focus on procedures instead of outcomes, writes Lokman Hossain in his blog post.
Checklist Parades, QMS Theater and Fee Pressures: ISO 9001 Systemic Issues
Drawing from his experience in auditing and quality management, Johnathon Grumelot, president of JAG Business Consulting, outlines several systemic issues he believes are inherent in ISO 9001.