News United States of America
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

FDA Aligns U.S. Device Rules With ISO 13485, Replaces QSR With QMSR
The U.S. Food and Drug Administration (FDA) has replaced its long-standing Quality System Regulation (QSR) with the new Quality Management System Regulation (QMSR), changing the legal framework for quality management in the U.S. medical device sector.
Winter Issue of ANSI in China Newsletter Highlights 2025 Developments
The American National Standards Institute (ANSI) has released the Winter 2025 edition of the ANSI in China Newsletter, outlining recent standards, policy, and regulatory developments linked to U.S. and China engagement.
Baldrige Foundation Announces 2026 Leadership Award Recipients
The Foundation for the Malcolm Baldrige National Quality Award has announced the 2026 recipients of its Leadership Awards,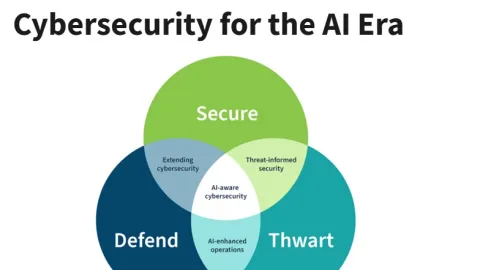
NIST Releases Draft on How Cybersecurity Programs Can Adapt to Artificial Intelligence
The National Institute of Standards and Technology (NIST) has released a preliminary draft that explains how organizations can adapt their cybersecurity programs to the growing use of artificial intelligence.
USPTO Creates Standard-Essential Patent Working Group Amid Licensing Disputes
The United States Patent and Trademark Office (USPTO) has created a new Standard-Essential Patent Working Group as disputes continue over how patented technologies built into technical standards are licensed and enforced.
Baldrige Performance Excellence Program Transitions to Two Private-Sector Partners
Beginning with the 2026 award cycle, most operational responsibilities for the Baldrige Performance Excellence Program will shift to two private-sector organizations, while federal authorities retain ownership and oversight of the framework and award process.
NIST Invites Comments on Guide Connecting Cybersecurity, ERM and Workforce Planning
The National Institute of Standards and Technology (NIST) has released the draft Special Publication 1308 2pd,
ANSI Sets Schedule for 2026 Standards Action Updates
The American National Standards Institute (ANSI) has released its 2026 Standards Action publishing calendar,
Quality Drives U.S. Manufacturing Resilience, ZEISS Report Finds
A new U.S. Manufacturing Insights Report 2025 from ZEISS Industrial Quality Solutions reveals that 93% of American manufacturers view quality assurance as mission-critical to overcoming industry challenges and maintaining competitiveness.
ANSI Honors 20 Leaders Advancing U.S. Standardization and Conformity Assessment
The American National Standards Institute (ANSI) recognized 20 outstanding professionals for their leadership in standards, conformity assessment, and workforce development during its 2025 Leadership and Service Awards Banquet on October 22.
U.S. Report Tracks Progress on 3D Printing Standards Gaps
America Makes and the American National Standards Institute (ANSI) released the September 2025 Gaps Progress Report,
Standards That Help Tackle the Growing Problem of Trash in Space
As Earth’s orbit becomes increasingly crowded with defunct satellites, rocket parts, and collision fragments, the need for global standards to manage space debris has become urgent.Global News

Work Begins on Two Agrifood Data System Standards
The International Organization for Standardization (ISO) has started work on two new international standards for agrifood data systems through ISO/TC 347, focused on crop data definitions and data exchange in greenhouse production.
ISO 17024 Enters Final Publication Stage
The revision of ISO/IEC 17024, the international standard that sets requirements for bodies certifying persons, has entered its final publication stage, with release expected in March 2026.
MDSAP Updates Audit Approach Toward Risk-Based Assessments
The Medical Device Single Audit Program (MDSAP) has updated its audit approach, marking a notable shift in how medical device manufacturers are evaluated across participating regulatory jurisdictions.
AIAG–VDA Release Harmonized SPC Yellow Volume for Automotive Industry
The Automotive Industry Action Group (AIAG) and the German Association of the Automotive Industry (VDA) have released a joint Statistical Process Control (SPC) Manual.
IAF Adds ISO 14067 Product Carbon Footprint To Its Multilateral Recognition Arrangement
The International Accreditation Forum (IAF) has decided to include ISO 14067 in its Multilateral Recognition Arrangement (IAF MLA), extending mutual recognition to
BRCGS Publishes FAQ For Packaging Materials Issue 7
BRCGS has released a new frequently asked questions (FAQ) document to support the implementation of the Global Standard Packaging Materials Issue 7.
GLOBALG.A.P. IFA Version 6 Audits Now Available for Combinable Crops and Plant Propagation Material
GLOBALG.A.P. has confirmed that audits are now available under Integrated Farm Assurance (IFA) version 6 for combinable crops and plant propagation material, two product categories within the IFA certification framework.
LEAF Updates Its Marque Standard With Version 17.0 For Sustainable Agriculture
LEAF (Linking Environment And Farming) has launched version 17.0 of the LEAF Marque Standard, introducing updated requirements for sustainable agriculture as the scheme continues to expand globally.
Revised ISO 2859-1 Brings Formal Skip-Lot Rules and New Curve Methods
The third edition of ISO 2859-1 has been published by the International Organization for Standardization (ISO), marking the first major revision of the international acceptance sampling standard in 27 years.
ISO 42119 Series Signals New Era for AI Testing and Assurance
The ISO/IEC 42119 series sets out a new international framework for testing and assuring artificial intelligence (AI) systems, with one document now published and several further parts under development.
ISO 14001 Revision Nears Publication After Final Draft Reaches Ballot Stage
The revision of ISO 14001, the international standard for environmental management systems, is nearing publication,

